In the last article I showed how the temperature change between Glacial and interglacial was sufficient to cause up to 2.3km of crust to be forced down into the earth’s core. In this article I examine how this could affect climate.
See also:
CO2 production from Cement making
A windmill takes energy from the wind and in so doing it opposes its motion. To enable that, each windmills has several hundred tonnes of concrete make from cement which is in turn produced by heating limestone rock.
The breakdown of calcium carbonate from heat is called thermal decomposition and the equations for this thermal decomposition of calcium carbonate are:
calcium carbonate ![]() calcium oxide + carbon dioxide
calcium oxide + carbon dioxide
This decomposition will occur at depths of around 100km or greater. So, although no ice-age cycle will be sufficient on its own to push crust down this far, the successive expansion and contraction will ratchet the rocket downward so that the rock moves another 2.3km downward toward the point where it decomposes.
Similar processes will occur for most carbon containing rocks so that overall up to 2.3km of the 40,000 km of crust will be pushed down.
Carbon is found throughout the earth but particularly in Marine sediments and rocks as shown by the following table.
What is the effect of this Thermal subduction?
When carbon bearing rock like limestones are heated in the earth’s crust they will give off CO2 and various other chemicals including water. Whilst the crust is not pushed down far at any plate, the net effect of the thermal expansion is to push around a kilometer of rock down which in turn pushes rock that was previous subducted during a previous cycle even further. The net result is that around 1km of rock will be heated to release its carbon and other products.
Warming
During the warming phase, the earth’s crust will expand pushing outward. With no where else to go, the rock will be forced down at so called subduction zones. (fig 4.2)
Thus at and after the warming phase from the interglacial we should expect to see an increase in earthquakes as the earth “creaks” much like a house creaks on a hot day as it heats or cools. (fig 4.3)
And whilst the 20th century warming is very short in comparison to the ice-age cycle some studies have shown a linkage:
- Another story about global warming causing volcanoes…
- CO2 causes earthquakes, is there anything it can’t do?
- Guardian: Global warming to trigger “earthquakes, tsunamis, avalanches and volcanic eruptions.”

Fig 4.3 Eventually pressure forces denser oceanic plate down beneath continental plate causing earthquake as plate moves downward.
As I explain above, the surface rock subducted will decompose releasing CO2 and other decomposition products like SO2 and water vapour. (Fig 4.4)

Fig 4.4 Oceanic crust is forced downwards where it is heated liberating CO2, water vapour, hydrocarbons and molten rock. These volatile components find their way upward toward vents where the expulsion of solid material with the various gases and water creates mound like hills called volcanoes.
And because it takes time for temperature changes at the surface to penetrate the crust, over time more and more crust is pushed down, eruptions continue until the expansion phase ceases and the remaining oceanic plate is decomposed at which point the eruptions cease.
Cooling
When the cooling phase sets in, the crust will begin to contract. However, rather than reversing the flow of mantel that has been subducted downward, it is far easier to find a week point of the crust and to pull it apart. Such weak spots exist at mid oceanic ridges such as that running down the centre of the Atlantic. Here the rock will be pulled apart (fig 4.5)
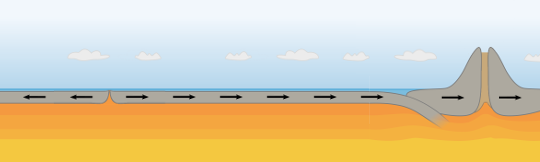
Fig 4.5 Start of Cooling Phase. The cooling surface entering an ice-age tends to cool the crust causing it to shrink. This leads to tensional forces.
So, the result of the cooling induced by the ice-age will be to increase the (apparent) rate of ocean floor spreading. (Fig 4.6)
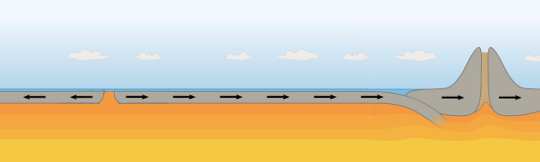
Fig 4.6 as the force increases, the crust splits, but instead of the slanting down thrust faults the cracks are vertical and tend to be where the crust is already thin from previous cycles from previous expansion events such as the centre of the Atlantic). Because the tensional strength of rock is low, these do not give rise to such significant earthquakes. Magma enters the expanding cracks.
Allowing fresh mantel material to fill the gap. (Fig 4.7) Material which does not contain all the volatile components that bubble up near the subduction zones.

Fig 4.7 Over time the molten magma solidifies at the spreading faults creating new crust bringing the cycle full circle ready to start the next warming phase.
And on to the next warming phase.
By filling the gap, the newly extruded crust material now prevents expansion, so that a further warming phase of the ice-age cycle, will have a ratchet effect subducting further material.
In effect, the crust moves like a caterpillar … first thermal expansion pushes material down subduction zones, then them al contraction pulls the tail up so that ice-age cycle by cycle the crust moves rather in the way of a caterpillar.
Evidence for a link between CO2 and ocean crust formation
Because this theory predicts a regular cycle of ocean crust formation corresponding to the ice-age cycle, I have been searching for evidence to support (or refute) this hypothesis. Now at the very last minute comes this from WUWT

Fig 4.8 Bathymetric and ice-age cycle (CO2) data normalized to a maximum amplitude of 1, and
superimposed
The prediction I am making is that ocean floor formation will be a minimum as we leave an ice-age (corresponding to lowest CO2) and then rise after thermal expansion to a maximum. However, whilst the above graph shows a correlation between sea floor formation and ice-age cycle, the sea floor parameter is depth of the sea and the age is an estimate which seems to be based on constant rates of sea floor spreading and the age is based on changes in magnetism through a single period of normal magnetic polarity.
So, whilst this is indicative of a correlation it is unclear but Tolstoy seems to believe it supports this hypothesis:
Maya Tolstoy:
Sea floor eruption rates, and mantle melting fuelling eruptions, may be influenced by sea-level and crustal loading cycles at scales from fortnightly to 100 kyr. An ~100kyr periodicity in fast-spreading sea floor bathymetry, and relatively low present-day eruption rates, at a time of high sea-level and decreasing orbital eccentricity suggest a longer term sensitivity to sea-level and orbital variations associated with Milankovitch cycles. Sea floor spreading is considered a small but steady contributor of CO2 to climate cycles on the 100 kyr time scale, however this assumes a consistent short-term eruption rate. Pulsing of sea floor volcanic activity may feed back into climate cycles, possibly contributing to glacial/inter-glacial cycles, the abrupt end of ice ages, and dominance of the 100 kyr cycle.
[Note: when I wrote this, I was expecting to use CO2 in a novel way to drive the climate. As I said in I’m now a CO2 denier, this approach ran into problems when I found CO2 and climate are not correlated during the cooling phase of the ice-age cycle. However, just as CO2 is released so other decomposition products will be released by this volcanic activity many of which are active in the climate. So the basic premise that the 100,000 year time delay for climate causing the ice-age cycle still has merit even if CO2 might not be the key ingredient being released]
Estimate of CO2 released
if we look at the amount of Carbon in the various constituents of the earth we find:
| Sink |
Amount in Billions of Metric Tons
|
| Atmosphere |
578 (as of 1700) – 766 (as of 1999)
|
| Soil Organic Matter |
1500 to 1600
|
| Ocean |
38,000 to 40,000
|
| Marine Sediments and Sedimentary Rocks |
66,000,000 to 100,000,000
|
| Terrestrial Plants |
540 to 610
|
| Fossil Fuel Deposits |
4000
|
Table 4.1 Source: Forest Hydrology: An introduction to Water and Forests
As we showed above, the expansion of the earth’s crust over an ice-age cycle at the surface is around 2.3km at the surface over 40,000km. Thus on average, the fraction of rock that would be forced down is given by:
Fraction of crust subducted x quantity of carbon in rocks.
= 2.3/40,000 x 100,000,000 billion tonnes
=6 x 1012
This means that if the subduction depending on the surface expansion, up to 6 x 1012 tonnes of Carbon would be released as a result of thermal expansion induced subuction after an ice-age. This is equivalent when released as CO2 to around 20,0o0 billion tonnes of CO2. In comparison:
Since 1750 humanity has added 520 billion metric tons of greenhouse gases to the atmosphere and we’re on pace to add that much again within 40 years
Even if we take the expansion at 2.3km down where the effect of the cooling takes longer to impact and so is smaller, the total CO2 released is the same as all the CO2 released by humanity so far.
CO2 isn’t the only game in town
However, whilst we have measurements of CO2, we must not forget that CO2 is not the only climate driver from volcano activity. Sulphur dioxide, particles and water vapour could all play their part.
This suggests that each glacial cycle will create around
2.3 x 100,000,000 / 40,000 billion tonnes of carbon in rocks
=6 x 1012 tonnes of carbon
=2 x 1013 tonnes of CO2
Summary
So, thermal expansion of the earth’s crust and subsequent decomposition of rock to release CO2 or some other components appears to have the magnitude of impact that might cause or trigger climate change and it occurs on roughly the time scale we require.
But even though masses of CO2 are released, it cannot on its own cause this change.
[Note: when I wrote this, I was expecting to use CO2 in a novel way to drive the climate. As I said in I’m now a CO2 denier, this approach ran into problems when I found ghat the level of CO2 is not correlated with temperature during the cooling phase of the ice-age cycle. There is a critical dip in temperature around 16,000 years after the inter-glacial peak without the necessary similar sized corresponding dip in CO2. So CO2 cannot be either directly responsible or causal via any linear feedback mechanism.
However, just as CO2 is released so other decomposition products will be released by this volcanic activity many of which are active in the climate. So the basic premise that the 100,000 year time delay for climate causing the ice-age cycle still has merit even if CO2 might not be the key or only ingredient being released]
See also:
High-Resolutio
n Phylogenetic Analysis of Southeastern Europe Traces Major Episodes of Paternal Gene Flow Among Slavic Populations
Lovorka Barać Lauc*, 1 ,
Irena Martinović Klarić*,
Siiri Rootsi†,
Branka Janićijević*,
Igor Rudan‡§,
Rifet Terzić∥,
Ivanka Čolak¶,
Ante Kvesić¶,
Dan Popović*,
Ana Šijački#,
Ibrahim Behluli**,
Dobrivoje Đorđevi憆,
Ljudmila Efremovska††,
Đorđe D. Bajec#,
Branislav D. Stefanović#,
Richard Villems† and
Pavao Rudan*
+ Author Affiliations
*Institute for Anthropological Research, Amruševa 8, 10000 Zagreb, Croatia; †Estonian Biocentre, University of Tartu, Tartu, Estonia; ‡School of Public Health Andrija Štampar, University of Zagreb Medical School, Zagreb, Croatia; §University of Edinburgh Medical School, Edinburgh, Scotland; ∥Medical Faculty, University of Tuzla, Tuzla, Bosnia and Herzegovina; ¶Clinical Hospital Center “Bijeli Brijeg,” Mostar, Bosnia and Herzegovina; #Emergency Unit of Clinical Center of Serbia, Belgrade, Serbia and Montenegro; **Medical Faculty, University of Prishtina, Prishtina, Kosovo; and ††Medical Faculty, University of Skopje, Skopje, MacedoniaE-mail: mpericic@luka.inantro.hr
Accepted May 30, 2005.
Abstract
The extent and nature of southeastern Europe (SEE) paternal genetic contribution to the European genetic landscape were explored based on a high-resolution Y chromosome analysis involving 681 males from seven populations in the region. Paternal lineages present in SEE were compared with previously published data from 81 western Eurasian populations and 5,017 Y chromosome samples. The finding that five major haplogroups (E3b1, I1b* (xM26), J2, R1a, and R1b) comprise more than 70% of SEE total genetic variation is consistent with the typical European Y chromosome gene pool. However, distribution of major Y chromosomal lineages and estimated expansion signals clarify the specific role of this region in structuring of European, and particularly Slavic, paternal genetic heritage. Contemporary Slavic paternal gene pool, mostly characterized by the predominance of R1a and I1b* (xM26) and scarcity of E3b1 lineages, is a result of two major prehistoric gene flows with opposite directions: the post-Last Glacial Maximum R1a expansion from east to west, the Younger Dryas-Holocene I1b* (xM26) diffusion out of SEE in addition to subsequent R1a and I1b* (xM26) putative gene flows between eastern Europe and SEE, and a rather weak extent of E3b1 diffusion toward regions nowadays occupied by Slavic-speaking populations.
Key words
phylogenetic analysis
Y chromosomal binary haplogroups
southeastern Europe (SEE)
Introduction
Southeastern Europe (SEE) has traditionally been viewed as a “bridge” (Childe 1958) between the Near East and temperate Europe or as a key area in the process of transition from hunter-gathering to agropastoral, farming societies in Europe (e.g., Ammerman and Cavalli-Sforza 1984; Renfrew 1987; Zvelebil and Lillie 2000). Recent phylogeographic analyses of Y chromosome E and J haplogroups indicate that southern Europe and the Balkans indeed could have been both the receptors and sources of gene flow during and after the Neolithic (Cruciani et al. 2004; Semino et al. 2004). The STR haplotype diversity of these two haplogroups is considerably younger than that of other Y chromosome haplogroups spread in Europe. Among the latter, haplogroup I, perhaps, most clearly represents the paternal genetic component of the pre-Neolithic Europeans. In contrast to E and J, haplogroup I is virtually absent in Middle East and West Asia (Semino et al. 2000), and two of its major subclades have frequency peaks in northern Balkans and Scandinavia (Rootsi et al. 2004). Semino et al. (2000) and Barać et al. (2003) hypothesized that, besides southwest Europe, the northern Balkans could have been another possible Last Glacial Maximum (LGM) refugium and a reservoir of M170.
In this study we first examined the extent and nature of SEE paternal genetic contribution to the European genetic landscape based on a high-resolution Y chromosome typing involving 681 unrelated males from four modern states, Croatia, Bosnia and Herzegovina, Serbia and Montenegro (including the province of Kosovo), and Macedonia (fig. 1). Second, we exploited available data on Y chromosome variation among different southern, western, and eastern Slavic-speaking populations in Europe to draw conclusions about possible origin of major paternal lineages in the Slavic gene pool. Finally, based on geography, we assessed patterns of Y chromosome diversity across SEE.
Materials and Methods
We analyzed 681 males from seven populations from SEE and 5,017 Y chromosomes from 81 western Eurasian populations available from literature. Blood samples were collected from healthy unrelated adults after obtaining informed consent. DNA was extracted using the salting-out procedure (Miller, Dykes, and Polesky 1988).
The following set of biallelic markers was analyzed using restriction fragment length polymorphism (RFLP) or in/del assays according to published protocols: M9 (Whitfield, Sulston, and Goodfellow 1995), YAP (Hammer and Horai 1995), SRY-1523 (Whitfield, Sulston, and Goodfellow 1995) (SRY-1523 is equivalent to SRY10831 [Whitfield, Sulston, and Goodfellow 1995]), 92R7 (Mathias, Bayés, and Tyler-Smith 1994), 12f2 (Rosser et al. 2000), M170, M173, M89 (Underhill et al. 2000), and P37 (Y Chromosome Consortium 2002). The polymorphic single nucleotide polymorphism (SNP) underlying markers M26, M35, M67, M69, M78, M81, M82, M92, M102, M123, M172, M201 (Underhill et al. 2000), M223 (Underhill et al. 2001), M241, M242, M253 (Cinnioğlu et al. 2004), and SRY8299/4064 (Whitfield, Sulston, and Goodfellow 1995) were sequenced after polymerase chain reaction (PCR) amplification. PCR-amplified products were purified using shrimp alkaline phosphatase and exonuclease treatment following Kaessmann et al. (1999) and sequenced using the BigDye Terminator Version 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, Calif.) on an ABI PRISM 310 Genetic Analyzer (Applied Biosystems) by using the DNA Sequencing Analysis Software Version 3.7 (Applied Biosystems). M9 was typed on all samples, and other markers were typed hierarchically according to their known phylogeny. A tentative assignment of all R1 chromosomes derived at M173 but without the G to A back mutation at SRY10831 into haplogroup R1b was based on the observations of Cruciani et al. (2002). Phylogenetic relationships of analyzed biallelic markers are presented in figure 2. Mutation labeling follows the Y Chromosome Consortium (2002).
Y chromosomal SNP tree and haplogroup frequencies (percent) in seven SEE populations. *Croatian mainland from Barać et al. (2003) was additionally genotyped for deeper resolution of I in Rootsi et al. (2004) and for E and J in the present study. E3b1α chromosomes were defined by A7.1 nine-repeat allele.
In addition, we surveyed eight short tandem repeats (STRs) DYS19, DYS385, DYS389I, DYS389II, DYS390, DYS391, DYS392, and DYS393 (Kayser et al. 1997) on all 681 SEE chromosomes and one additional GATA STR A7.1 (DYS460) (White et al. 1999) in E3b1-M78 chromosomes. PCR products were detected on an ABI PRISM 310 Genetic Analyzer (Applied Biosystems), and fragment sizes were analyzed by the GeneScan Analysis Software Version 3.7 (Applied Biosystems).
Expansion ranges were expressed as the age of STR variation estimated as the average squared difference in the number of repeats of seven STRs (DYS19, DYS389I, DYS389II, DYS390, DYS391, DYS392, and DYS393) between all sampled chromosomes and the founder haplotype divided by w (effective mutation rate of 0.00069 per locus per 25 years) (Zhivotovsky et al. 2004). Phylogenetic networks were obtained by using the same seven STRs as those used for expansion range estimates. The phylogenetic relationships between microsatellite haplotypes were determined by using the program NETWORK 4.0b (Fluxus Engineering). Networks were calculated by the median-joining method (Bandelt, Forster, and Röhl 1999), and STR loci were weighted according to Helgason et al. (2000). Haplogroup-frequency and haplogroup-variance surfaces were reconstructed following the Kringing procedure by use of the Surfer System (Golden Software), the frequency data reported in table 1 and variance data from this study and literature, as specified in figures 3–7. Credible regions (95% CRs) for haplogroup frequencies were calculated from posterior distribution of the proportion of the group of lineages in the population, as in Richards et al. (2000). For the purpose of correlating Y chromosomal frequencies with geography, we used Spearman’s bivariate correlation procedure (SPSS for Windows, 7.5.1.). Sampled individuals were pooled into 12 regional towns (fig. 1) with following latitude (N) and longitude (E) values: (1) 46°02′, 15°90′; (2) 45°82′, 15°98′; (3) 45°77′, 18°17′; (4) 45°40′, 14°80′; (5) 45°23′, 13°93′; (6) 42°65′, 18°09′; (7) 44°22′, 17°90′; (8) 43°35′, 17°80′; (9) 43°39′, 17°55′; (10) 44°82′, 20°46′; (11) 42°67′, 21°17′; and (12) 41°98′, 21°43′.
Summarized Percent Frequencies of R1b, R1a, I1b* (xM26), E3b1 and J2e
I1b* (xM26) frequency and variance surfaces in SEE (panels A and B) were generated from the data in this study. I1b* (xM26) frequency surfaces in Europe, northern Africa, and Asia Minor (panel C) were generated from the data reported in table 1, and variance surfaces (panel D) were generated from STR data in this study and Rootsi et al. (2004).
E3b1 frequency and variance surfaces in SEE (panels A and B) were generated from the data in this study. E3b1 frequency surfaces in Europe, northern Africa, and Asia Minor (panel C) were generated from the data reported in table 1, and variance surfaces (panel D) were calculated from STR data in this study and Semino et al. (2004).
R1a frequency and variance surfaces in SEE (panels A and B) were generated from the data in this study. R1a frequency surfaces in Europe, northern Africa, and Asia Minor (panel C) were generated from the data reported in table 1, and variance surfaces (panel D) were calculated from STR data in this study, Rootsi et al. unpublished data, Cinnioğlu et al. (2004), Behar et al. (2003), Weale et al. (2002), Wilson et al. (2001), Helgason et al. (2000), and Hurles et al. (1999). Shaded areas in panel D correspond to regions for which combined SNP and STR Y chromosomal data are not available.
R1b frequency and variance surfaces in SEE (panels A and B) were generated from the data in this study. R1b frequency surfaces in Europe, northern Africa, and Asia Minor (panel C) were generated from the data reported in table 1, and variance surfaces (panel D) were calculated from STR data in this study, Rootsi et al. unpublished data, Cinnioğlu et al. (2004), Behar et al. (2003), Weale et al. (2002), Wilson et al. (2001), Helgason et al. (2000), and Hurles et al. (1999). Shaded areas in panel D correspond to regions for which combined SNP and STR Y chromosomal data are not available.
J2e frequency and variance surfaces in SEE (panels A and B) were generated from the data in this study. J2e frequency surfaces in Europe, northern Africa, and Asia Minor (panel C) were generated from the data reported in table 1, and variance surfaces (panel D) were generated from STR data in this study and Semino et al. (2004).
Results and Discussion
One-third of the studied SEE Y chromosomes has the derived P37 C allele and is classified to haplogroup I1b* (xM26) (fig. 2). A detailed survey demonstrates that I1b* (xM26) lineages reach maximum frequency in SEE (fig. 3C) and that I1b* (xM26) STR variance peaks over a large geographic region encompassing both southeastern and central Europe (fig. 3D). I1b* (xM26) frequency peaks in Herzegovinians (64%) and Bosnians (52%) while preserving substantial (30%) frequencies in all SEE populations with the exception of two reproductively isolated and non-slavic speaking populations, Kosovar Albanians and Macedonian Romani (fig. 3A). The incidence of I1b* (xM26) decreases from SEE toward western (from 20% in Slovenians abruptly to 1% in northern Italians) and southern (17%–18% in Albanians and northern Greeks, 8% in southern Greeks, 2% in Turks) and retains frequencies of 7%–22% in central and eastern Europe (table 1). The highest STR variance of I1b* (xM26) lineages (0.34 to 0.23) is in Bosnians, Czechs and Slovaks, Hungarians, Herzegovinians, and Serbians (fig. 3B and D). In both cases, when all studied SEE populations are considered together and upon exclusion of Kosovar Albanians and Macedonian Romani, I1b* (xM26) frequency and variance do not show significant correlations with geography (table 2). Moreover, I1b* (xM26) phylogenetic network (fig. 8A) shows high haplotype diversity and sharing of founder haplotype among investigated populations. In fact, homogenous distribution of elevated frequency accompanied with high diversity of I1b* (xM26) lineages among different SEE populations may be viewed as a genetic signature of their common paternal history over a long period of time. Rootsi et al. (2004) estimated that I1b* (xM26) diverged from I* at 10.7 ± 4.8 kilo years ago (KYA), possibly relating to the post–Younger Dryas (YD) climate amelioration in Europe, and that I1b* (xM26) expansion occurred around the early Holocene at 7.6 ± 2.7 KYA. Considering only our SEE sample, the coalescent estimate of I1b* (xM26) is substantially older (11.1 ± 4.8 KYA). This finding suggests that the I1b* (xM26) lineages might have expanded from SEE to central, eastern, and southern Europe, presumably not earlier than the YD to Holocene transition and not later than the early Neolithic.
Correlations of Major Y Chromosome Haplogroup Frequencies and Variances with Geography
Microsatellite networks of major Y chromosomal lineages in SEE: (A) I1b* (xM26) (B) E3b1α; (C) R1a. Microsatellite haplotypes are represented by circles, with areas proportional to the number of individuals harboring the haplotype. Smallest circle represents single haplotype in panel B and C and two haplotypes in panel A. Branch lengths are proportional to the number of one-step mutations separating two haplotypes.
Haplogroup E3b1-M78 is the second most prevailing one (23%) in the studied sample with E3b1-M78 chromosomes accounting for almost all E representatives (98%) except a single E3b2-M81 and two E3b3-M123 chromosomes (fig. 2). E3b1-M78 is the most common haplogroup E lineage in Europe (Cruciani et al. 2004; Semino et al. 2004). The spatial pattern shown in figure 4(C) depicts a nonuniform E3b1 geographic distribution with a frequency peak centered in south Europe and SEE (13%–16% in southern Italians and 17%–27% in the Balkans). Declining frequencies are evident toward western (10% in northern and central Italians), central, and eastern Europe (from 4% to 10% in Polish, Russians, mainland Croatians, Ukrainians, Hungarians, Herzegovinians, and Bosnians). Noteworthy is a low E3b1 frequency (5%) in Turkey. Apart from its presence in Europe and the Middle East, E3b1 is also found in eastern and northern Africa. Cruciani et al. (2004) estimated that E3b-M78 might have originated in eastern Africa about 23.2 KYA (95% confidence interval [CI] 21.1–25.4). Although present level of phylogenetic resolution does not allow further subdivision of this haplogroup by binary markers, based on strong geographic structuring of diverse microsatellite motifs, E3b-M78 is suggested to be a collection of subclades with different evolutionary histories (Cruciani et al. 2004; Semino et al. 2004) out of which the α cluster, largely characterized by an A7.1 nine-repeat allele, is confined to Europe (the Balkans) and Turkey (Cruciani et al. 2004). E3b1 variance distribution depicted in figure 4(D) does not overlap with its frequency distribution possibly because analyzed E3b1 chromosomes harbor diverse background motifs. It is very likely that a variance peak centered in northeastern Africa as well as high variance values in Turkey and southern Italy are due to the inclusion of δ (and a few southern Italian β) chromosomes. Almost 93% of SEE E3b1 chromosomes are classified into α cluster. In Europe, the highest E3b1α variance is among Apulians, Greeks, and Macedonians, and the highest frequency of the cluster is among Albanians, Macedonians, and Greeks (table 1). Bearing in mind the congruent E3b1α frequency, variance maximums, and star-like phylogenetic network (fig. 8B), it is possible to envision that a yet undefined sublineage downstream of M78, characterized by the nine-repeat allele at A7.1 locus, may have originated in south Europe and SEE from where it dispersed in different directions. Furthermore, it may be envisioned that the observed E3b1α frequency distribution in Anatolia might stem from a back migration originating in south Europe and SEE. Our estimated range expansion of 7.3 ± 2.8 KYA is close to the 7.8 KYA (95% CI 6.3–9.2 KYA) estimate for expansions of cluster α chromosomes in Europe reported by Cruciani et al. (2004) and the 6.4 KYA estimate for E3b1-M78 STR variance in Anatolia dated by Cinnioğlu et al. (2004). The frequency and variance decline of E3b1 in SEE is rather continuous (fig. 4A and B), with a frequency peak extending from the southeastern edge of the region and a variance peak in southwest. Observed high E3b1 frequency in Kosovar Albanians (46%) and Macedonian Romani (30%) represent a focal rather than a clinal phenomenon resulting most likely from genetic drift. E3b1 frequency and variance are significantly correlated with latitude, showing higher values toward south (table 2), both when all SEE populations are considered (r = −0.51, P = 0.05, for frequency and r = −0.706, P = 0.05, for variance) and when Kosovar Albanians and Macedonian Romani are excluded (r = −0.597, P = 0.05, for frequency and r = −0.676, P = 0.05, for variance). A lower frequency of E3b1 significantly distinguishes populations of the Adriatic-Dinaric complex, i.e., mainland Croatians, Bosnians, and Herzegovinians (7.9%; 95% CI 0.054–0.114), from their neighboring populations of the Vardar-Morava-Danube river system, i.e., Serbians and Macedonians (21.9%; 95% CI 0.166–0.283). These observations hint a mosaic of different E3b1 dispersal modes over a short geographic distance and point to the Vardar-Morava-Danube river system as one of major routes for E3b1, in fact E3b1α, expansion from south and southeastern to continental Europe. In fact, dispersals of farmers throughout the Vardar-Morava-Danube catchments basin are also evidenced in the archaeological record (Tringham 2000).
R1a haplogroup occurs at 16% frequency in SEE (fig. 2). The age of M17 has been approximated to 15 KYA (Semino et al. 2000; Wells et al. 2001). Kivisild et al. (2003) suggested that southern and western Asia might be the source of R1 and R1a differentiation. Current R1a-M17/SRY-1532 distribution in Europe shows an increasing west-east frequency and variance gradients with peaks among Finno-Ugric and Slavic speakers (fig. 5C and D). Similar to I1b* (xM26), R1a frequency gradient decreases slowly to the south (to 10% in Albanians, 8% in Greeks, and 7% in Turks) and abruptly in the west (3% in Italians) (table 1). R1a frequency and STR variance decrease in the north-south direction in SEE, from 34%–25% in mainland Croatians and Bosnians to 12%–16% in Herzegovinians, Macedonians, and Serbians (fig. 5A and B). Moreover, R1a frequency is significantly correlated with latitude (table 2) when all studied SEE populations are considered (r = 0.865, P = 0.01) and also when Kosovar Albanians and Macedonian Romani are excluded (r = 0.743, P = 0.01). High R1a haplotype diversity in SEE is evident in the phylogenetic network (fig. 8C) and the estimated range expansion at 15.8 ± 2.1 KYA, consistent with its deep Paleolithic time depth, as previously suggested (Semino et al. 2000; Wells et al. 2001). At this level of resolution, it is not clear what temporal and effective population size differences contributed to this deep Paleolithic signal as high R1a variance in SEE might be explained by either ancient demography or more recent bottlenecks and founder effects in different Slavic tribes. At least three major episodes of gene flow might have enhanced R1a variance in the region: early post-LGM recolonizations expanding from the refugium in Ukraine, migrations from northern Pontic steppe between 3000 and 1000 B.C., as well as possibly massive Slavic migration from A.D. 5th to 7th centuries.
R1b haplogroup is present in SEE at a level of 9% (fig. 2). R1b-M173 lineages are considered to trace an Upper Paleolithic migration from West Asia to European regions then occupied by Aurignacian culture (Semino et al. 2000; Underhill et al. 2001; Wells et al. 2001). The spatial distribution of R1b lineages shows a frequency peak (40%–80%) in western Europe and a decrease in eastern (with the exception of 43% in the Ossetians) and southern Europe (fig. 6C), whereas R1b variance shows multiple peaks in West Europe and Asia Minor (fig. 6D). While R1b variance displays a clear-cut northwestern-southeastern decline in SEE (fig. 6B), R1b frequency decline continues from western toward southeastern and southern Europe, but two intermediate local peaks are evident, in north among mainland Croatians and Serbians and in south among Kosovar Albanians, Albanians, and Greeks (fig. 6C). These spatial patterns might be due to the fact that R1b lineages contain associated RFLP 49a,f ht 15 and 35 sublineages with opposite distributions possibly reflecting repeopling of Europe from Iberia and Asia Minor during the Late Upper Paleolithic and Holocene (Cinnioğlu et al. 2004). The overall R1b frequency distribution in the Balkan Peninsula suggests its possible arrival from two different source populations during recolonization of Europe. We estimated the range expansion of R1b lineages in SEE at 11.6 ± 1.4 KYA. Although R1b lineages could have accumulated STR variance before diffusion in SEE, it is significant that its estimated range expansion almost perfectly matches the coalescent estimate for the I1b* (xM26) lineages, pointing to the YD to Holocene transition as possibly a period when these two major Y chromosome lineages started to expand in the region.
Haplogroup J defined by a 12f2 polymorphism is subdivided into two major clades, J1-M267 and J2-M172 (Cinnioğlu et al. 2004). J2-M172 is more prevalent in Europe where at least five different lineages can be traced—J2e*-M102, J2e1-M241, J2*-M172, J2f*-M67, and J2f1-M92 (fig. 2, Semino et al. 2004). In SEE, the most frequent are J2e lineages that comprise 5% of all chromosomes, while J2f cluster, a predominant J2 cluster in Greeks and Italians (Di Giacomo et al. 2004), is present at a frequency less than 1% (fig. 2). Most likely due to genetic drift, Kosovar Albanians harbor a J2e frequency peak whereas variance maximum declines from the southeastern edge of the studied region (fig. 7A and B). Even though J2e frequencies do not correlate with geography, J2e variances show significant correlations with latitude and longitude and are highest toward south and east of the region (table 2). The correlation between geography and haplogroup frequencies are significant when all SEE populations are considered (r = −0.949, P = 0.05) and when Kosovar Albanians and Macedonian Romani are excluded (r = −0.949, P =0.05). Our estimated range expansion for J2e at 2.8 ±1.6 KYA (for all SEE populations) and 3 ± 1.9 KYA (SEE populations without Kosovar Albanians) succeeds the dates of 7.9 ± 2.3 KYA (Semino et al. 2004) and 8.6 KYA (Cinnioğlu et al. 2004). The J2e-M102 spatial distribution depicted in figure 7(C and D) with two frequency and variance peaks positioned in the Balkans and central Italy may be explained by the maritime spread of J2e lineages from southern Balkans toward Apennines at times later than those based on the classical model of demic expansions carried by Neolithic agriculturists from the Middle East via Balkans toward rest of Europe.
Widely spread Romani haplogroup H1 is a major lineage cluster in Macedonian Romani (fig. 2). A 2-bp deletion at M82 locus defining this haplogroup was also reported in one-third of males from traditional Romani populations living in Bulgaria, Spain, and Lithuania (Gresham et al. 2001). Its ancestral M52 A → C transversion was reported in the Vlax Roma (Kalaydjieva et al. 2001) and India (Ramana et al. 2001; Wells et al. 2001; Kivisild et al. 2003). Out of 34 H1-M82 males, 10 were typed for mitochondrial DNA (mtDNA) and belonged to haplogroup M that was highly frequent in Macedonian Romani (Cvjetan et al. 2004), traditional Romani populations (Gresham et al. 2001), and India (Kivisild et al. 2003). High prevalence of Asian-specific Y chromosome haplogroup H1 and mtDNA haplogroup M supports their Asian (Indian) origin and a hypothesis of a small number of founders diverging from a single ethnic group in India (Gresham et al. 2001).
F*, G-M201, K* (xP), P* (xR1, Q), and Q-M242 lineages occur at low frequencies in SEE (fig. 2). The Herzegovinian Q-M242 sample harbors a STR motif previously seen in eastern Adriatic haplogroup Q lineages that are marked by the typical presence of the unusually long DYS392-15 allele (Barać et al. 2003).
We conclude that even though the majority of identified SEE paternal lineages are consistent with the typical European Y chromosome gene pool, their distribution and estimated range expansions clarify the specific role of this region in structuring the European genetic landscape. Contemporary Slavic paternal gene pool is characterized by the predominance of R1a and I1b* (xM26) variants as well as the scarcity of E3b1 lineages as a result of the following prehistoric gene flows. First, we envision the post-LGM R1a expansion from eastern to western Europe and second the YD-Holocene I1b* (xM26) diffusion out of the Balkans in addition to subsequent R1a and I1b* (xM26) putative gene flows between eastern Europe and SEE. Lastly, we envision a weaker extent of E3b1 dispersal out of southern Europe and SEE toward eastern Europe rather than toward western (especially Mediterranean) Europe. Our results also stress that I1b* (xM26) wide geographic distribution and massive frequencies accompanied with high diversity in most of its range among major SEE populations testify impressively to their common paternal history, whereas observed genetic heterogeneity structured mostly along the northwestern-southeastern axis is a result of attested prehistoric and historical gene flows with different temporal and directional characteristics. Yet the main difference between the paternal genetic history of the Slavic-speaking populations lies in the presence, among eastern Slavs (Russians, Ukrainians, Belarussians), of haplogroup N chromosomes, virtually absent among any of the western or southern Slavic populations (Rosser et al. 2000; Semino et al. 2000; Barać et al. 2003; Tambets et al. 2004), unequivocally suggesting that the historic eastward expansion of Slavs in the middle of the first millennium A.D. resulted in a substantial admixture of them with the substratum populations, inhabiting East Europe, among whom this largely northern Eurasian haplogroup was and still is widely spread.
Acknowledgments
We are grateful to all the donors for their kind participation in this study. Special thanks go to Toomas Kivisild for friendly guidance and helpful comments for this manuscript. We wish to express our gratitude to two anonymous reviewers for their helpful suggestions. This research was supported by the Ministry of Science, Education and Sports of the Republic of Croatia grant for project 0196005 (to P.R.), Estonian basic research grant 514 (to R.V.), European Commission Directorate General Research grant ICA1CT20070006 (to R.V.), and Estonian Science Foundation grant number 6040 to Kristiina Tambets.
Footnotes
↵1 The first two authors contributed equally to this study.
Lisa Matisoo-Smith, Associate Editor
References
Articles citing this article


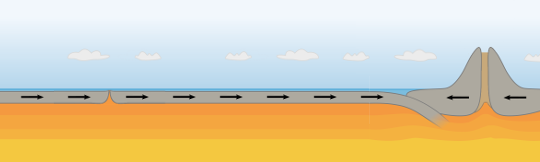









Pingback: Toward a new theory of ice-ages VIII (How CO2 could control climate) | Scottish Sceptic
Tolstoy has been barking about this since 1992, and most folks think she is wrong. Willis Eschenback has several posts debunking her latest paper in a convincing fashion over at WUWT.
You are certainly right about subduction zones recycling CO2 from its sequestered carbonate state. In fact, there are credible estimates (google) that with out this volcanic recycling, life would cease because CO2 would fall to the point where plant photosynthesis would fail in about 2.5my.
But there is AFAIK no geological evidence for any periodicy whatsoever in volcanism. And there are many places along the Pacific ring of fire where sufficiently deep geological cores have been taken that would show this if it were true. You might want to dig around some more than I did.
Rud, as far as I can see Tolstoy is talking about tides and volcanoes. This is very different from what I am proposing which is thermal expansion over periods of 40-100,000 years.
It’s a simple matter of physics that the earth’s crust must expand and contract over the ice-age cycle. This isn’t anything that anyone can reasonably dispute. And it’s a simple fact that this expanding rock must go somewhere or that the stresses will built up – making earthquakes more likely.
And there is very good evidence this is occurring in the following way:
1.There is evidence of ocean crust formation changing on an ice-age cycle as shown above.
2. And there is evidence for a massive release of CO2 from the ice-core.
3. There is no other tenable explanation for the 40-100k ice-age cycle except the very long time it takes for the crust to expand and contract.
Pingback: Toward a new theory of ice-ages VII (hitting the buffers) | Scottish Sceptic
Pingback: The Caterpillar theory of tectonic plate movement – it’s just simple physics. | Scottish Sceptic
I agree that the effect of warming and cooling on the expansion and contraction of the Earth’s crust is a factor that is overlooked in theories of the cause of the Ice Ages.
Also overlooked is:
The effect of the Earth cooling and shrinking
You say the shrinking will be taken up by the mid-ocean cracks reducing.. I think the shrinking causes the ridges at right –angles to the vertical cracks.
There is also the long-term shrinkage caused by the long-term cooling of the hot core.
According to Le Chatelier’s Principle, a system reacts to oppose change.
A sphere will tend to a shape that has least surface to cool from.
The shape with least surface area is a tetrahedron. The Earth is cooling to a tetrahedron.
The land is at the corners and edges, the oceans are in the depressed faces.
Looking from N: land surrounding the Arctic Ocean
Looking from S: Southern Ocean surrounding the Antarctic Land and 3 continents pointing to it
Google Tetrahedral Earth theory, especially the brilliant 1875 book by your fellow Scottish sceptic William Lothian Green
Geothermal gradient
Also interesting is your fig.4.1. Where is this from?The underground lapse rate T/h is 10C/km near the surface but get less as the depth increases. This is not expected if the source is the hot centre. The value is similar to the T/h of the atmos. I suggest both are caused by gravity T/h = g/c. Surprisingly specific heat of rocks and air are similar.
“There is also the long-term shrinkage caused by the long-term cooling of the hot core.”
That’s a very good point!! Seeing the way I didn’t even think about that, I will have to be less critical of those who didn’t think about cooling of the crust.
The geotherm is quite a common graph. In my rust I didn’t credit the source. It may have gone from this URL http://archive.eps.mq.edu.au/courses/GEOS260/SHIELD~1.JPG which is now given a “forbidden” so I can’t check or credit them.
Check my website tectonicforces.org for a similar discussion of how the Milankovitch periodicities are the driving force for making the Earth’s plates move.
Jon Thoreau Scott