Simply
The temperature of the earth as seen from space is the average temperature (or more accurately average heat energy) of all the molecules and surfaces emitting IR to space. If this average temperature increases more IR is emitted and the earth cools. If this average temperature decreases, less IR is emitted and the earth warms.
The temperature of molecules is determined by the lapse rate which means the temperature in the lower atmosphere drops by about 6.5C/km.
If there are more molecules present in the atmosphere like CO2 which are active in the IR, then they tend to block IR from lower levels and cause more emission from higher levels. In effect the average radiation height increases and therefore IR is emitted from colder levels, less IR energy is emitted and so the earth warms up.
We can calculate the change in IR caused by a doubling of CO2 in the atmosphere. This amounts to change in average radiation level of about 150m.
Introduction
Over the years I have seen many people arguing about how the Greenhouse effect works and from these discussions it is clear that most people misunderstand it. That is not to say that adding a greenhouse gas like CO2 to a typical atmosphere like the earth’s should not cause warming. Instead, whilst this “greenhouse” warming is a nice & simple way to explain to an average person on street how increasing CO2 would cause warming it is not true any other sense than it is what tends to happen and is certainly not a “law” of nature.
Yes it works to indicate what is happening, unfortunately as soon as people start talking about the detailed mechanisms behind it or worse using it to predict behaviour, this simple explanation fails and cause a lot of senseless argument because it does not and cannot encompass the true complexity of the situation.
So, this guide is intended to go one step beyond the simple “man on the street” explanation.
Greenhouse gases are not really “greenhouses”
It’s indicative that this “greenhouse gas theory” is not the best science when I have to start by saying that the mechanism by which greenhouse warm plants is not at all the same physical mechanism as we are discussing here. The reality is that whilst the sun both warms a greenhouse like the earth, the greenhouse works by physically restraining the gases so that the hot gases are kept within the confines of the greenhouse.
Greenhouse Gas “theory”
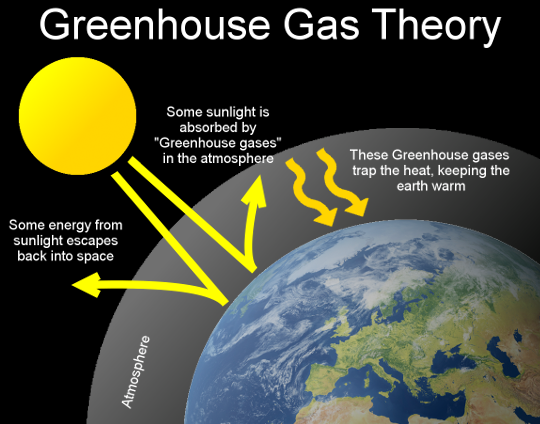
Typically websites will show diagrams like the one below labelling this mechanism as a “theory” as if it were based on some fundamental law of nature. So let’s just recap this “theory”.
Shown above is the normal outline of the “Greenhouse gas” theory. The sun is shown shining on the earth through the atmospheric layer. The energy flowing in then disperses either heading back into space or as shown to the top, being “trapped” by greenhouse gases which then “back radiate” the trapped heat down to the earth’s surface.
As a qualitative description of what happens, this illustrates many of the key energy flows, but it is clearly not quantitative. Such descriptions seldom if ever show any kind of equations, formulas or any other mechanism by which any of these flows can be calculated or justified. They appear to come from nowhere, their meaning is obscure and as such they are in effect hand-waving arguments which give little if any scientific insight to what is actually happening.
They are at best illustrative, at worst misleading.
False Criticisms based on Laws of Thermodynamics
The most often heard criticism raised against this depiction of the energy flow, is that “warming” is said to occur as a result of back radiation of heat trapped by greenhouse gases. This requires heat to move from a colder region (the atmosphere) to the warmer surface of the earth. This is felt to break the second law of thermodynamics as it is often stated:
the second law of thermodynamics says that heat flows naturally from an object at a higher temperature to an object at a lower temperature, and heat doesn’t flow in the opposite direction of its own accord.
However, the heat flow is actually from the surface to the air and back again and the net flow when all things are considered is from the hotter surface to the colder atmosphere. It is not that heat is flowing from the colder area to warmer, but that the colder object is reducing the flow away from the hotter (much as a coat or blanket will be colder than a person, but reduces the flow from away from them to the environment)
The physical basis of CO2 interaction with IR.
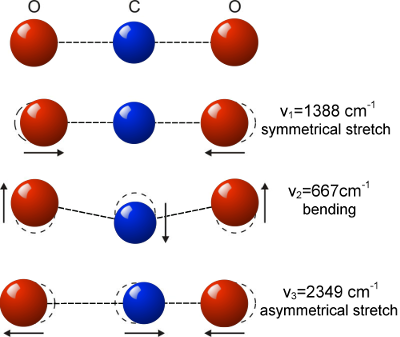
In order for molecules to interact with electromagnetic radiation like IR, they require to have a charge dipole. That is to say, the average position of positive charges must be offset to that of the average position of negative charges. Looking at the diagram to the right showing the normal vibrational modes, it is clear that Carbon dioxide doesn’t have a molecular dipole in its ground state where the carbon is mid-way between the two oxygen atoms. However, some CO2 vibrations where the oxygen and carbon atoms move in different directions do produce a structure with a molecular dipole.
Looking at the diagram, a symmetrical stretch where both oxygen atoms move in opposition to each other leaves the average position of all oxygen atoms unchanged. Therefore the vibration associated with this at 1388cm-1 does not interact with electromagnetic radiation. However where the oxygen atoms move perpendicular to the axis in the bending vibration, their average position moves relative to the carbon and so the associated wavelength of 667cm-1 interacts strongly. Likewise, where the central carbon moves back and forth toward each oxygen we get strong interaction at 2349cm-1 .
Because of this, CO2 strongly interacts with infra-red radiation. This interaction can be seen in the spectrum of radiation leaving the earth where there are obvious “holes” where the spectrum is deficient at various frequencies. The CO2 hole can be clearly seen around 667cm-1 as well as those for other gases.

Absorption spectra of electro-magnetic radiation leaving the earth’s surface with red line representing spectrum of idealised black body.
Two way energy flow into greenhouse gases
Having a vibration mode where the average positive and negative charges allows CO2 to absorb radiation as below:
However, but addition when the CO2 molecule is vibrating that dipole moment acts like a radio antenna and so it can also emit radiation.
So, the very physical property that enables a molecule to absorb radiation at the wavelength of its vibrational modes is also the same physical property which means that if it already is vibrating, then it can release this energy as electromagnetic radiation.
To show that these must be equal, consider an infinite universe with nothing else but CO2 molecules all at the same temperature. The chance of 667cm-1 radiation being emitted must be exactly the same chance of it being absorbed. Therefore at the same temperature, the probability of absorption is the same as emission. Therefore if a gas is an absorber of radiation, then** it is also an emitter of that same radiation.
**at least for a simple molecule like CO2
Blackbody radiation
According to the Stefan Boltzmann principle, a body at of uniform temperature radiates energy per unit of area according to the equation:
E/A = εσT4
Where E is energy flow (Watts), A is area of body (m2), σ is Stefan Boltzmann constant (
~5.67x 10-8 Wm-2K-4), T is temperature (Kelvin) and ε is a fraction which ranges between zero and one. ε varies between surfaces and depends on a variety of factors from chemical make up to topology of the surface and the range of wavelengths being considered. In general, the more complex the chemical composition of the surface and the more complex the topology, the higher the value of ε.
Emissivity in Surfaces
Because those surfaces which most readily give off radiation at any frequency will usually be equally able to absorb radiation at the same frequency, in most cases the value of ε in the above equation is also the fraction of incoming electromagnetic radiation at the same frequencies that will be absorbed. So for light incident on a surface as shown right for an incident electromagnetic flow E onto the surface, the absorption will be:
Eabsorbed = εE
And therefore as the remainder has to go somewhere, it will be reflected so:
Ereflected = (1-ε)E
Emissivity in Gases
Like a surface, a gas also has an emissivity corresponding to the fraction of light it will absorb and that which it will not. However, unlike a surface, a gas molecule is too small to reflect the non absorbed light and instead the light that is not absorbed but diffracts around the molecule giving equations:
Eabsorbed = εE
Enon-absorbed = (1-ε)E
How does greenhouse warming work?
To explain the mechanism of greenhouse warming, we are going to have the simplest possible planet and atmosphere. The planet is a uniform body and it’s atmosphere consists of a single molecule of CO2 (if necessary consider it embedded in an otherwise inert gas with no absorption or emissions at the wavelengths of interest)
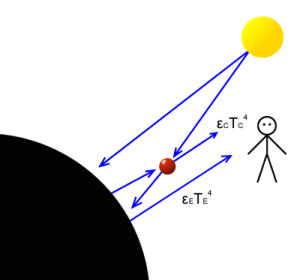
Energy flows for our one molecule atmosphere showing position of observer looking toward earth past the single CO2 molecule.
Here we have a planet with a sun (yellow), which for simplicity we will consider to be a point source. It shines onto the planet (black) as well as the single molecule of CO2 (red).
Everything is at thermal equilibrium with the planet at temperature TE and the CO2 molecule at TC.
According to the Stefan Boltzmann principle, a body radiates energy per unit of area according to the equation:
E/A = εσT4
And so I have marked on the corresponding energy flows that would be seen by an observer away from the earth.
Viewed from the observer, the earth would look as the diagram to the right. One side of the huge circular shape of the earth visible in full except for one part obscured by the single CO2 molecule. And for our purposes, we will assume the CO2 molecule is so close to the earth that:
- Either: It obscures the earth from the observer
- Or the earth obscures the CO2 molecule
How does this single molecule affect the temperature of the earth?
(1) Heat flow per unit area from earth (E) = εEσTE4
(2) Heat absorbed by CO2 per unit area = εCE
(3) Non absorbed heat flowing past CO2 per unit area = (1-εC)E
(4) Heat emitted from CO2 per unit area = εCσTC4
Combining (1) and (3):
(5) Non absorbed heat past CO2 per area = (1-εC) εEσTE4
So total heat flow out from and past CO2 molecule (per unit area) is:
(6) EC = (1-εC) εEσTE4 + εCσTC4
So the change in heat flow caused by the CO2 (per unit area) is flow from earth – heat flow past CO2:
δE = EC – E
(7) δE = ( (1-εC) εEσTE4 + εCσTC4 ) – εEσTE4
Simplifying we get:
δE = εEσTE4 – εC εEσTE4 + εCσTC4 – εEσTE4
(9) δE = εCσTC4 – εC εEσTE4
So if δE is positive, then the CO2 molecule has caused a net increase in heat flow from the earth and so there is a net cooling effect and if δE is negative, then heat flow from the earth has decreased and there is a net warming effect. So (eliminating σ):
[ εCσTC4 – εC εEσTE4 ] > 0 ⇒ Global Cooling
[ εCTC4 – εC εEσTE4 ] = 0 ⇒ Stasis
[ εCTC4 – εC εEσTE4 ] < 0 ⇒ Global Warming
Rearranging and removing σ, the condition for global warming is that:
(10) TC4 / εETE4 < 1
or
(11) ( TC / TE ) < εE 0.25
and so if
TC < TE εE 0.25 ⇒ Global Warming
The earth’s emissivity is given as ocean: 0.90 to 0.93 (link) and land surface 0.96 (link). Assuming 70% of the planet is sea, this gives an average emissivity of approximately
εE = (0.7 *(0.9+0.93)/2) + 0.3 * .96 ~= 0.93
So if the average surface temperature (TE) is 15C (288K), then we arrive at the following:
TC < 288 x 0.930.25
TC < 282K (9C)
Based on a planet with the earth’s average emissivity and a surface temperature of 15C but unlike the earth, with a uniform surface temperature, then the addition of one molecule of CO2 would depend on its temperature as follows:
TC > 9C ⇒ Global Cooling
TC = 9C ⇒ Stasis
TC < 9C ⇒ Global Warming
Is CO2 a global warming gas?

The threshold between CO2 causing warming and cooling for the average emissivity of the earth ( ε=0.93)
As we can see from the calculations above, adding a gas that interacts with infra-red has an affect on the atmosphere, but that affect does not necessarily lead to global warming. Whether or not there is global warming is affected by the temperature of the atmosphere (and earth’s surface emissivity/albedo).
Radiative Height
By now you should understand that the key thing that is changing the outgoing radiation is the change in the temperature of the molecules which in turn changes by their height in the atmosphere. The temperature profile is due to the lapse rate which is in effect the change of heat energy to potential energy. (In order to lift up the mass of air, energy must come from somewhere).
Having looked at at an atmosphere with one molecule we can extend this simply to an atmosphere by saying that the average temperature of a planet as viewed from space is the average temperature (or to be more precise average heat energy) of all the last molecules or surfaces that emitted radiation into space. But where are these?
We can work this out because the situation for emission is the same as absorption. In other words, if we were to shine an “IR torch” down into the atmosphere, the IR would be gradually absorbed as it passed down. And the emission curve will be identical. Below is plotted a series of emission/absorption curves for our atmosphere showing how it changes as the proportion of greenhouse gases change:
 Except at the ground surface, the curves are for all practical purposes the same. As such the effect of changing greenhouse gas concentration is merely to raise of lower the entire curve by a fixed amount. The scale of this change is not large and would be about 150m for a doubling of CO2.
Except at the ground surface, the curves are for all practical purposes the same. As such the effect of changing greenhouse gas concentration is merely to raise of lower the entire curve by a fixed amount. The scale of this change is not large and would be about 150m for a doubling of CO2.
But don’t forget those white fluffy things called clouds
However, before finishing this article, I would just like to mention those things we call clouds. The above analysis is based on a highly unrealistic planet without clouds. Clouds have a significant effect on earth as they change the albedo of the earth from around 0.93 to 0.7. This would then mean that for CO2 to cause warming, the temperature of that CO2 needs to be below:
TC < 288 x 0.70.25
TC < 263K (-10C) ⇒ global warming
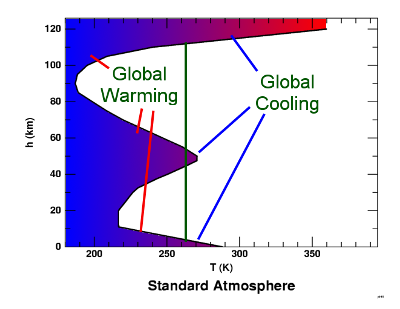
The threshold between CO2 causing warming and cooling for an albedo of 0.3 ( ε = 0.7 i.e. earth with clouds)
Now, much more of the lower atmosphere where the atmosphere is more dense could cause cooling (albeit much is below cloud layer). Also we start to see cooling layers at 50km as well as the more of the very upper atmosphere.
So, now there are three zones that cause cooling. Moreover, if we then start to consider warmer air moving poleward, there will be significant areas where the CO2 is causing active cooling of the planet in total contradiction to the common assertion that CO2 always and inherently causes warming.







Pingback: Understanding the Global Temperature V – Met Balloon Data | Scottish Sceptic
Pingback: The Science | Scottish Sceptic
Hi Mike
I don’t think you have this part quite right. The incoming short wave radiation from the Sun is not being absorbed/emitted by the atmosphere.
The incoming radiation hits the ground which is absorbed causing Brownian motion (work) which causes longwave infrared radiation to be emitted and it is this that is absorbed and remitted by greenhouses gasses causing the greenhouse effect…as I understand it.
Regardless of this, Greenhouse gasses are typified by their high emissivity. Increasing the proportion of greenhouse gasses in the atmosphere increases it’s emissivity which will increase the emissivity of the atmosphere. If you want to insulate anything you decrease it’s emissivity, it’s ability to achieve equilibrium with it’s environment.
Proof of this is seen in the mid Tropospheric hotspot which turned out to be a coolspot, which is exactly what you would expect to see if greenhouse gasses increase the emissivity of the atmosphere and cause it to cool.
Cheers
Damian thanks. Yes the heat is supplied by the incoming visible radiation, but the way to understand the greenhouse effect, is that the earth is stable when the average outgoing radiation temperature is that of the blackbody. So, if you average the temperature of all outgoing radiation it has to be equal to the blackbody temperature.
The temperature of the various layers is created by the flow of heat through the atmosphere via convection because not much travels via IR in the atmosphere as it is so dense (except in the radiative windows). It’s only in the upper atmosphere that IR loss becomes dominant, which is why you need to think about the greenhouse effect from the top down and not the way many try to describe it which is from the bottom up.
Pingback: When theory hits the buffer – maybe CO2 does not have an effect? | Scottish Sceptic
Pingback: Predicting the surface temperature on other planets | Scottish Sceptic
What about methane ? Its 33times stronger than Co2 in its effect on global warming. And its “production” also increases year by year, as the animal herds we need for our meat supply increase rapidly.
You do realise that you can experience the whole “catastrophic” effect of increasing CO2 by climbing down a small hill. Indeed, father Christmas would experience close to the “massive” average decadal change in temperature just by climbing down a chimney – although obviously that change due to air pressure, like the “global warming” temperature change we experienced until 2000, was swamped by other things other than the small changes due to greenhouse gases.
That is a great start to a realistic model. When you add in clouds, you also need to consider latent heat transport to the lower condensation level, this naturally decreases lapse rate and increases convection..which is the introduction of chaos.
Thanks. Yes I agree that lapse is a complexity all of its own – and one totally ignored by standard model.
I like the model as it works at several levels. You can make it very simple and suggest “the radiation leaves at a level approximately at the tropopause or 5km. A lapse rate of 6.5km therefore means the greenhouse temperature is 6.5 x 5km = 32.5C. But you can make it more complex by looking at the range of heights from which radiation is emitted, and the average (T^4) temperature. Or you can go even further and consider the probability of emission at a range of heights for each “window” in the spectrum.
In many instances, because the effective radiation curve moves up and down together, you can think about it in terms of height. Or you can think of the dual model with part of the radiation coming from the surface and the rest from the atmosphere (so the average T^4 must be the black body temperature). The good thing is by focussing on what leaves the planet, it completely side steps the very complex heat transfer mechanism in the lower atmosphere which can be replaced with the simple to understand idea of lapse rate.
The point where the model becomes less predictive and more qualitative is in bands with a substantial percentage of outgoing radiation coming from the surface, but you could modify with a correction. The other thing it hides is that the lapse rate also to some extent involves a radiation flux. But that is a relatively small effect and for a reasonable first approximation can be ignored.
Obviously, it doesn’t replace a layer-by-layer detailed model – but as a conceptual introduction I think it is far better and more physically realistic than the “heat trapping” model that gets used.
If this average temperature increases more IR is emitted and the earth cools. If this average temperature decreases, less IR is emitted and the earth warms…
very discouraging to read that in the first paragraph..
more IR or not, the earth cools due to emitting IR ,at all times…
if more IR is admitted , the earth is warmer globally..
if less IR is admitted the earth is cooler globally…
I think you’re missing the point about “more” & “less” IR. Like putting on a coat, it doesn’t make you warmer instead it allows LESS or MORE heat to escape from your body.
Indeed, the effect is not that dissimilar. Because when you put on a thick coat, whilst you are a lot warmer on the inside, because the coat is preventing heat reaching the outside of the coat, it become cooler. So a cooler surface of the coat means a warmer person inside the coat. Likewise a cooler earth as observed from space would mean that heat was being trapped. That heat trapping would continue until the surface of the earth as viewed from space returns to normal (due to warming).
To help you understand how cooling the outer surface helps trap heat (or trapping heat results in a cooler outer surface, the same are the same) here is a video: https://youtu.be/zDUgzRVPKns?t=1m31s
An excellent article Scottish-Sceptic. I need to consider it in more depth.
Amid the radiation calc. Lies the wild card of water. For every kilogram of water evaporated from the surface some 680 WattHrs are dissipated into the atmosphere at various heights, with a proportion winding up in the cirrus clouds some 12 kilometres up there nudging the tropopause as ice crystals.
However at around 238 deg.K these crystals still emit radiation into space as they dendicitly grow until gravity takes a hold.
No idea how that affects the radiation calcs. Across the various levels.
My regards.
In the troposphere, IR flux is an important driver, but as we all know the temperature effect is swamped by convection which stabilises the lapse rate. So whilst the “mickey mouse” model of IR flux from the surface isn’t wrong – it doesn’t even mention lapse rate. What I’ve done is include this key mechanism. And by talking about the greenhouse effect in terms of lapse rate, I’m hiding all the complexity of water circulation and low level IR flux within a single parameter. In fact that parameter is enormously complex. But lapse rate is at least measurable and simple to understand & within a range predictable, even if what drives it and the exact value due to water circulation is incredibly complex.
Quite right Scottish-. Very complex. However the fact remains that due to water being lighter than dry air the latent heat is carried up and dissipated way past the majority of the CO2 by reduction of the optical path. The energies involved rather knock the socks off pesky CO2. And then, of course, gravity plays it’s part and we have a Rankine Cycle in operation which tends to circulate faster should more energy be introduced rather than increasing in temperature.
In no way am I criticising your radiative analysis, particularly as I have not yet considered it in detail. All I am doing is offering a different perspective which I think probably reinforces your conclusions.
It’s not different – it’s just that I don’t spell it out. The point with this approach of looking from the outside in as to what gets out, rather than the surface “looking up and seeing what comes back”, is that the radiation leaving the earth is emitted from all surfaces & molecules “visible” from space in that particular spectrum. And if that surface/molecule happens to be water vapour in a cloud it is treated the same as CO2. The only question is which is higher.
I’ve occasionally said that this approach would come to the same as any good model – but by good I mean including a detailed understanding of the cloud formation process and the heat transfer. The way I approach it, you don’t need to understand that – instead it is all included in the father simple idea of “lapse rate” and a height (range) at which radiation leaves the planet.
Pingback: The connection between Global Warming and the earth’s core temperature | Scottish Sceptic
So much of what is taken as true or false in the natural sciences has to do with what is easy to understand and convey. Climatology and its most perverse theory of catastrophic global warming is only the most obvious variant on this theme. Misinformation and blatant pseudoscience is thick in all of the natural sciences. At the root of it all is a brain-dead, artificially simplistic understanding of H2O. And the silence of fools.
Much of the foolishness in the natural sciences started with Linus Pauling, about 60 or 70 years ago, when he made a conceptual error and the rest of the scientific community blindly followed. I refer to this error as Pauling’s Omission.
Here is a link to a video that obviates this error and its wider ramification:
Pauling’s Omission: The Original Sin of the Natural Sciences
https://www.youtube.com/edit?o=U&video_id=iIQSubWJeNg
Pingback: Molecule Vibration - Chemical Literature