Over the years I have seen many people arguing about how the Greenhouse effect works and from these discussions it is clear that most people misunderstand it. That is not to say that adding a greenhouse gas like CO2 to a typical atmosphere like the earth’s should not cause warming. Instead, whilst this “greenhouse” warming is a nice & simple way to explain to an average person on street how increasing CO2 would cause warming it is not true any other sense than it is what tends to happen and is certainly not a “law” of nature.
Yes it works to indicate what is happening, unfortunately as soon as people start talking about the detailed mechanisms behind it or worse using it to predict behaviour, this simple explanation fails and cause a lot of senseless argument because it does not and cannot encompass the true complexity of the situation.
So, this guide is intended to go one step beyond the simple “man on the street” explanation.
Greenhouse gases are not really “greenhouses”
It’s indicative that this “greenhouse gas theory” is not the best science when I have to start by saying that the mechanism by which greenhouse warm plants is not at all the same physical mechanism as we are discussing here. The reality is that whilst the sun both warms a greenhouse like the earth, the greenhouse works by physically restraining the gases so that the hot gases are kept within the confines of the greenhouse.
Greenhouse Gas “theory”
Typically websites will show diagrams like the one below labelling this mechanism as a “theory” as if it were based on some fundamental law of nature. So let’s just recap this “theory”.
Shown above is the normal outline of the “Greenhouse gas” theory. The sun is shown shining on the earth through the atmospheric layer. The energy flowing in then disperses either heading back into space or as shown to the top, being “trapped” by greenhouse gases which then “back radiate” the trapped heat down to the earth’s surface.
As a qualitative description of what happens, this illustrates many of the key energy flows. But it is clearly not quantitative such descriptions seldom if ever come with figures and none ever show any kind of equation, formulas or any other mechanism by which any of these flows can be calculated.
It is at best illustrative, at worst potentially misleading.
A False Criticisms
The most often heard criticism raised against this depiction of the energy flow, is that “warming” is said to occur as a result of back radiation of heat trapped by greenhouse gases. This requires heat to move from a colder region (the atmosphere) to the warmer surface of the earth. This is felt to break the second law of thermodynamics as it is often stated:
the second law of thermodynamics says that heat flows naturally from an object at a higher temperature to an object at a lower temperature, and heat doesn’t flow in the opposite direction of its own accord.
However, the heat flow is actually from the surface to the air and back again and the net flow when all things are considered is from the hotter surface to the atmosphere.
The physical basis of CO2 interaction with IR.
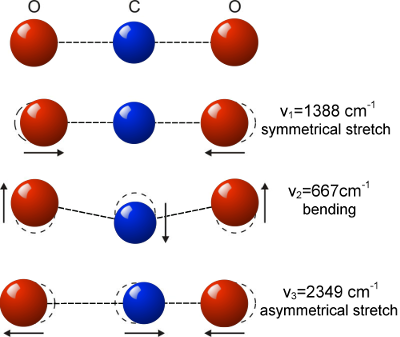
In order for molecules to interact with electromagnetic radiation like IR, they require to have a charge dipole. That is to say, the average position of positive charges must be offset to that of the average position of negative charges. Looking at the diagram to the right showing the normal vibrational modes, it is clear that Carbon dioxide doesn’t have a molecular dipole in its ground state where the carbon is mid-way between the two oxygen atoms. However, some CO2 vibrations where the oxygen and carbon atoms move in different directions do produce a structure with a molecular dipole.
Looking at the diagram, a symmetrical stretch where both oxygen atoms move in opposition to each other leaves the average position of all oxygen atoms unchanged. Therefore the vibration associated with this at 1388cm-1 does not interact with electromagnetic radiation. However where the oxygen atoms move perpendicular to the axis in the bending vibration, their average position moves relative to the carbon and so the associated wavelength of 667cm-1 interacts strongly. Likewise, where the central carbon moves back and forth toward each oxygen we get strong interaction at 2349cm-1 .
Because of this, CO2 strongly interacts with infra-red radiation. This interaction can be seen in the spectrum of radiation leaving the earth where there are obvious “holes” where the spectrum is deficient at various frequencies. The CO2 hole can be clearly seen around 667cm-1 as well as those for other gases.

Absorption spectra of electro-magnetic radiation leaving the earth’s surface with red line representing spectrum of idealised black body.
Two way energy flow into greenhouse gases
Having a vibration mode where the average positive and negative charges allows CO2 to absorb radiation as below:
However, but addition when the CO2 molecule is vibrating that dipole moment acts like a radio antenna and so it can also emit radiation.
So, the very physical property that enables a molecule to absorb radiation at the wavelength of its vibrational modes is also the same physical property which means that if it already is vibrating, then it can release this energy as electromagnetic radiation.
To show that these must be equal, consider an infinite universe with nothing else but CO2 molecules all at the same temperature. The chance of 667cm-1 radiation being emitted must be exactly the same chance of it being absorbed. Therefore at the same temperature, the probability of absorption is the same as emission. Therefore if a gas is an absorber of radiation, then** it is also an emitter of that same radiation.
**at least for a simple molecule like CO2
Blackbody radiation
According to the Stefan Boltzmann principle, a body at of uniform temperature radiates energy per unit of area according to the equation:
E/A = εσT4
Where E is energy (joules), A is area of body (m2), σ is Stefan Boltzmann constant (
~5.67x 10-8 Wm-2K-4), T is temperature (Kelvin) and ε is a fraction which ranges between zero and one. ε varies between surfaces and depends on a variety of factors from chemical make up to topology of the surface and the range of wavelengths being considered. In general, the more complex the chemical composition of the surface and the more complex the topology, the higher the value of ε.
Emissivity in Surfaces
Because those surfaces which most readily give off radiation at any frequency will usually be equally able to absorb radiation at the same frequency, in most cases the value of ε in the above equation is also the fraction of incoming electromagnetic radiation at the same frequencies that will be absorbed. So for light incident on a surface as shown right for an incident electromagnetic flow E onto the surface, the absorption will be:
Eabsorbed = εE
And therefore as the remainder has to go somewhere, it will be reflected so:
Ereflected = (1-ε)E
Emissivity in Gases
Like a surface, a gas also has an emissivity corresponding to the fraction of light it will absorb and that which it will not. However, unlike a surface, a gas molecule is too small to reflect the non absorbed light and instead the light that is not absorbed but diffracts around the molecule giving equations:
Eabsorbed = εE
Enon-absorbed = (1-ε)E
How does greenhouse warming work?
To explain the mechanism of greenhouse warming, we are going to have the simplest possible planet and atmosphere. The planet is a uniform body and it’s atmosphere consists of a single molecule of CO2 (if necessary consider it embedded in an otherwise inert gas with no absorption or emissions at the wavelengths of interest)
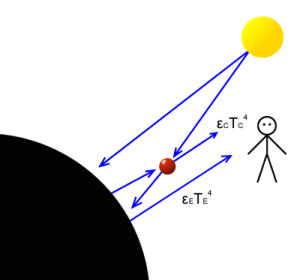
Energy flows for our one molecule atmosphere showing position of observer looking toward earth past the single CO2 molecule.
Here we have a planet with a sun (yellow), which for simplicity we will consider to be a point source. It shines onto the planet (black) as well as the single molecule of CO2 (red).
Everything is at thermal equilibrium with the planet at temperature TE and the CO2 molecule at TC.
According to the Stefan Boltzmann principle, a body radiates energy per unit of area according to the equation:
E/A = εσT4
And so I have marked on the corresponding energy flows that would be seen by an observer away from the earth.
Viewed from the observer, the earth would look as the diagram to the right. One side of the huge circular shape of the earth visible in full except for one part obscured by the single CO2 molecule. And for our purposes, we will assume the CO2 molecule is so close to the earth that:
- Either: It obscures the earth from the observer
- Or the earth obscures the CO2 molecule
How does this single molecule affect the temperature of the earth?
(1) Heat flow per unit area from earth (E) = εEσTE4
(2) Heat absorbed by CO2 per unit area = εCE
(3) Non absorbed heat flowing past CO2 per unit area = (1-εC)E
(4) Heat emitted from CO2 per unit area = εCσTC4
Combining (1) and (3):
(5) Non absorbed heat past CO2 per area = (1-εC) εEσTE4
So total heat flow out from and past CO2 molecule (per unit area) is:
(6) EC = (1-εC) εEσTE4 + εCσTC4
So the change in heat flow caused by the CO2 (per unit area) is flow from earth – heat flow past CO2:
δE = EC – E
(7) δE = ( (1-εC) εEσTE4 + εCσTC4 ) – εEσTE4
Simplifying we get:
δE = εEσTE4 – εC εEσTE4 + εCσTC4 – εEσTE4
(9) δE = εCσTC4 – εC εEσTE4
So if δE is positive, then the CO2 molecule has caused a net increase in heat flow from the earth and so there is a net cooling effect and if δE is negative, then heat flow from the earth has decreased and there is a net warming effect. So (eliminating σ):
[ εCσTC4 – εC εEσTE4 ] > 0 ⇒ Global Cooling
[ εCTC4 – εC εEσTE4 ] = 0 ⇒ Stasis
[ εCTC4 – εC εEσTE4 ] < 0 ⇒ Global Warming
Rearranging and removing σ, the condition for global warming is that:
(10) TC4 / εETE4 < 1
or
(11) ( TC / TE ) < εE 0.25
and so if
TC < TE εE 0.25 ⇒ Global Warming
The earth’s emissivity is given as ocean: 0.90 to 0.93 (link) and land surface 0.96 (link). Assuming 70% of the planet is sea, this gives an average emissivity of approximately
εE = (0.7 *(0.9+0.93)/2) + 0.3 * .96 ~= 0.93
So if the average surface temperature (TE) is 15C (288K), then we arrive at the following:
TC < 288 x 0.930.25
TC < 282K (9C)
Based on a planet with the earth’s average emissivity and a surface temperature of 15C but unlike the earth, with a uniform surface temperature, then the addition of one molecule of CO2 would depend on its temperature as follows:
TC > 9C ⇒ Global Cooling
TC = 9C ⇒ Stasis
TC < 9C ⇒ Global Warming
Is CO2 a global warming gas?

The threshold between CO2 causing warming and cooling for the average emissivity of the earth ( ε=0.93)
As we can see from the calculations above, adding a gas that interacts with infra-red has an affect on the atmosphere, but that affect does not necessarily lead to global warming. Whether or not there is global warming is affected by the temperature of the atmosphere (and earth’s surface emissivity/albedo).
Conclusion
It is incorrect to state “CO2 is a greenhouse gas” as if this is some law of nature or suggest it is a “theory” that has been proved. Instead the effect of adding gases like CO2 which interact with infra-red is dependent on the temperature of that molecule. If the atmospheric temperature is low enough it tends to reduce outgoing radiation and warm the world, if however the temperature of the atmosphere is high enough it will tend to cause global cooling.
However, if we refer to the idealised temperature profile of the atmosphere shown above where I have drawn on a line at 282K, we see that except for the first couple of kilometres above the surface and over 110km where the addition of CO2 causes Global cooling. So it is true that:
practically in most parts of the atmosphere, CO2 causes warming
And that:
overall adding CO2 will tend to cause warming.
But don’t forget those white fluffy things called clouds
However, before finishing this article, I would just like to mention those things we call clouds. The above analysis is based on a highly unrealistic planet without clouds. Clouds have a significant effect on earth as they change the albedo of the earth from around 0.93 to 0.7. This would then mean that for CO2 to cause warming, the temperature of that CO2 needs to be below:
TC < 288 x 0.70.25
TC < 263K (-10C) ⇒ global warming
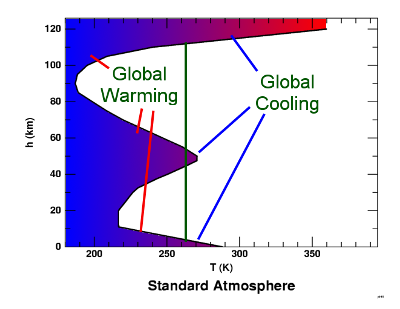
The threshold between CO2 causing warming and cooling for an albedo of 0.3 ( ε = 0.7 i.e. earth with clouds)
Now, much more of the lower atmosphere where the atmosphere is more dense could cause cooling (albeit much is below cloud layer). Also we start to see cooling layers at 50km as well as the more of the very upper atmosphere.
So, now there are three zones that cause cooling. Moreover, if we then start to consider warmer air moving poleward, there will be significant areas where the CO2 is causing active cooling of the planet in total contradiction to the common assertion that CO2 always and inherently causes warming.
Addendum
Thanks to mkelly for pointing out I had forgotten to add Stephan Boltzman constant.







From my layman’s position.
Have been following this discussion on Real science regarding a greenhouse. I have been trying to think of it more in terms of a lagged, hot water tank with heating element. When factoring in an in and out pipe for the cold and hot water this seems to change the picture and then when in reality there is no metal tank or lagging this would seem to represent reality so the hot body surrounded by a blanket confuses me. I can only assume that the slow movement of water over the heating element , as seen in nature- evaporation, would not allow a green house effect ( meaning a greenhouse where there is no use of water/wind to cool ) but a constant removal of heat . Of course the amount of humidity would dictate night time temp and of course the starting point for next days temp on Sun Up- so yes a GHG at night.
So when a hot body surrounded by a blanket analogy is used, would it not be better to assume that the blanket is in fact wet/ moist with moisture continually added – representing the atmosphere and would also allow the body to cool, a dry blanket to me would represent the desert.
When you are a layman you use layman’s words!
fascinating to think about.
Although a wet blanket is not the actual physical process – it is a good analogy in the sense that it could warm – but it could also cool.
Note my “atmosphere” only has one molecule and the earth’s has around 1×10^44, convection, clouds, a host of other gases as well as CO2.
But the one thing that I’m discovering more and more about this “greenhouse theory” is how unscientific it really is – and how it is quite complex to find any real science.
It is as if, having found some way to get the PR out that CO2 is a greenhouse gas, their interest in the “science” disappears.
Yes, you started with one molecule… so doubling that to two molecules will raise the planet’s temperature by .6 degrees, then adding two more will raise that another .6 degrees… Wow! I wonder how many doublings it takes to get to .04% of the atmosphere!
No, I am, of course, just joking, and pointing out that the so-called CO2 doubling factor is only a rule of thumb and has limits of usefulness.
My daughter really hated this “one molecule atmosphere” and absolutely insisted it wasn’t an atmosphere until it had at least a million molecules. I smuggling pointed out that if all these molecules were over the UK, there’d be an average spacing of 500m.
She then asked me how many there were … so I guessed a trillion trillion (10^24) and then we looked on line and found 1E44. – only out by a fact of 1E20 to 1!!! I didn’t feel so smug then!
All very well, but – just gaze into a Northern red sunset and wonder why then your retina do not fry………..
well there`s still ONE HUNDRED/m2 or so WATTS of ENERGY at around 0.8um lighting-up the sky whereas, in the LIR world, at 15umt (CO2`s blocking frequency) there is LESS THAN FIVE for Co2 to take a nibble at.
And so, when it comes to real heat, 0.8um at about 120c and 15um near 20c, 3why is it we BELIEVE that Co2 is any sort of a problem warming-wise and not cooling??
If like the IPCC you intentionally use out of date data for CO2 spectra, then multiply the result by some incredible positive feedback figure – you can if you are totally gullible come up with something that looks like a problem.
But if you start with the latest HITRAN data (like Hermann Harde) which gives 0.6C warming and if you understand that the climate cannot have positive feedbacks during an interglacial otherwise we’d never get the “full stop” to warming at the interglacials, then we are talking less than 0.2-0.6C warming during the next century +/- 1.0C** natural variation.
**estimate
Hard for the layman to grasp this one as well,
“This requires heat to move from a colder region (the atmosphere) to the warmer surface of the earth. This is felt to break the second law of thermodynamics as it is often stated”
I have two rooms, one heated one not. I open the door between them, obviously the temp in the unheated room dictates how much heat is lost from the heated room but the heat will travel from the heated to unheated until , hopefully, both are in equilibrium ( never happens in my house , both rooms just seen to end up cold!), but now as we have two rooms to heat the temp will be reduced in the heated room.
Where’s the water vapor? It’s all lot more than clouds and albedo.
IPCC AR5 TS.6 Key Uncertainties is where climate science “experts” admit what they don’t know about some really important stuff. They are uncertain about the connection between climate change and extreme weather especially drought. Like the 3” drought that hit Phoenix. They are uncertain about how the ice caps and sheets behave. Instead of gone missing they are bigger than ever. They are uncertain about heating in the ocean below 2,000 meters which is 50% of it, but they “wag” that’s where the missing heat of the AGW hiatus went, maybe. They are uncertain about the magnitude of the CO2 feedback loop, which is not surprising since after 17 plus years of rising CO2 and no rising temperatures it’s pretty clear whatever the magnitude, CO2 makes no difference.
http://www.writerbeat.com/articles/3713-CO2-Feedback-Loop
Barring some serious flaw in science or method, Miatello’s paper is the death certificate for AGW/CCC.
http://principia-scientific.org/publications/PSI_Miatello_Refutation_GHE.pdf
If a pound of air at 60 F and 20% RH (15.3 grains/lb dry air) absorbs water vapor through latent evaporation and increases its RH to 80% (61.9 grains/lb dry air), its heat content increases by about 8 Btu/lb. No sensible heat added, no dry bulb temperature change.
For that same pound of air at 60 F and 20% RH to absorb the 8 Btu/lb mentioned above without absorbing more water vapor the dry bulb temperature would have to increase by 30 F! (90F, 7.3% RH, same 15.3 grains/lb dry air)
Water vapor is a serious heat mover/absorber/releaser. Air is pathetic.
So the heat leaving the greenhouse does not equal the heat entering by the difference of the heat absorbed by the water vapor. During the night the process reverses, the water condenses, cooling and removing heat.
It’s the water vapor cycle that runs the climate, not CO2 & GHGs.
The molecule could just as well be water vapour. What I was mainly trying to do, was to show that the greenhouse effect is a symptom of how IR interactive molecules like CO2 and H20 behave in our atmosphere and that it is highly temperature depending whether they are warming or cooling gases so “greenhouse warming” is not some physical law. (Which is what one sceptic blogger has been suggesting).
And yes, water vapour is key, so is the convection cycles – but whilst not part of this model I added the bit on the end to say “hey there’s much more and it’s all to do with water”.
Hello Scottish Skeptic, just adding to this,
“So, if opening such a window did cause a significant drop in temperature this proves that heat is lost from convection”
I have quite a large greenhouse and can open the windows and door a notch, In the Summer to cool the greenhouse down I open these windows and doors a notch and open the windows in the much cooler room adjoining. I would expect this room to warm and the heat to disappear through here, in fact what happens is you an feel a drought immediately coming through the windows of the adjoining room into the greenhouse, I guess this is the ” Stack effect” What surprises me is it doesn’t happen the other way around , drought from green house to adjoining room and out.
( I have been banned from RS!)
Hot air is surprisingly reluctant to do what you think it should. For years we’ve had a cold hall/stairwell. I try to heat the place, but somehow the heat always rises up the stairs and cold comes down. Logically (being the centre of the house) it ought to be the warmest part of the house – but no. I’ve been round with an IR thermometer trying to work out why the hall is always cold downstairs, we’ve put on masses of insulation – and still all I know is that there’s a cold draught from upstairs and a corresponding rising one (the stairs have a low ceiling and you can feel the air moving).
The flow is caused by the difference in density which is a function of temperature like those tall smoke stacks at the power plants. Massive convection. Heat will not flow from the hotter greenhouse to the cooler room in opposition to the density.
cont…
knowing the difference in temp between the poles and tropics I wonder why we don’t have continual high winds but I am guessing that the Stack effect is made worse by air pressure in a building.
But we do – they are called the trade winds!
cont,
but this must illustrate that the notion of trapped gases causing runaway warming is a nonsense as this movement of air illustrates that the planet cools effectively from other means. My greenhouse soon cools!!
Not sure about this but I think there are so many air molecules hitting most CO2 molecules so often that re-radiation of IR can be neglected, i.e. the basic process is:
IR + CO2 + air –> CO2 + warmer air.
Of course, everything radiates IR all the time, but I suspect that the concept of back-radiation is misleading. What really makes the surface warmer than it would otherwise be is simply warmer air.
The fact that the air is colder than the surface is another red-herring, if anyone doesn’t believe that then go outside naked on a winters night, and compare it with the same on a summers night. (not in Scotland though, you’ll freeze both times!).
The key to changes in temperature is whether there is a change in incoming or outgoing radiation. For that you need to consider the earth from a point of view of an observer outside the earth.
“Back radiation” is only important in determining how that heat moves through the atmosphere. That is important for an observer on the earth – but this perspective is largely irrelevant when considering global warming.
Which begs the question: “Why do climate models look at the planet from the point of view of an observer on the ground?” Is this:
a) Because this is the best place to calculate the change in heat flows?
b) Because the climate academics are incompetent?
c) Because it produces better PR as journalists can’t understand anything unless they can see it from their own perspective?
So warm air warms the surface. Why is it then that the swimming pools in Phoenix, AZ have to be heated? The air is 105 F. Why doesn’t that heat the water in the pool? If the heater breaks, the pool will cool to maybe 65 – 70 F. Well, because evaporation cools the pool a lot faster than the air can heat it.
IPCC AR5 TS.6 Key Uncertainties is where climate science “experts” admit what they don’t know about some really important stuff. They are uncertain about the connection between climate change and extreme weather especially drought. Like the 3” drought that hit Phoenix. They are uncertain about how the ice caps and sheets behave. Instead of gone missing they are bigger than ever. They are uncertain about heating in the ocean below 2,000 meters which is 50% of it, but they “wag” that’s where the missing heat of the AGW hiatus went, maybe. They are uncertain about the magnitude of the CO2 feedback loop, which is not surprising since after 17 plus years of rising CO2 and no rising temperatures it’s pretty clear whatever the magnitude, CO2 makes no difference.
http://www.writerbeat.com/articles/3713-CO2-Feedback-Loop
Barring some serious flaw in science or method, Miatello’s paper should be the death certificate for AGW/CCC.
http://principia-scientific.org/publications/PSI_Miatello_Refutation_GHE.pdf
Pingback: Some thoughts on Greenhouse warming | Scottish Sceptic
“Why is it then that the swimming pools in Phoenix, AZ have to be heated?”
Large heat capacity of large bodies of water is why you have to heat swimming pools, and why it takes centuries for the ocean to warm in response to warmer air. You would not have to heat the pools if the nights were also 105F, but you would have to heat them if both days and nights were only 50 F.
Are you saying that warmer air does not keep the surface warmer than colder air? If so please explain why my body (always warmer than the air where I live) feels warmer in summer than winter.
You will always have to heat them because of the evaporation loss which doesn’t care about hot or cold, day or night, only how dry the air is.
The sensible heat capacity of water is 1 Btu/lb-F, but the latent heat of evaporation/condensation is about 1,000 Btu/lb.
Because your body loses heat faster. The body is 98.6 F. If outside is 75 F, that’s a 23.6 F difference. If outside is 30 F, that’s a difference of 68.6 F. The rate of heat loss is proportional to the temperature difference. Your body also loses moisture which is part of wind chill effect. Objects don’t care about wind chill, but your body does.
try wearing light wool , in Africa tribes wear wool to keep the bodies cool in the searing heat.
Or a black dish dash.
Mike, please sit down, I am going to say something that will surprise you..
The presence of an atmosphere makes earth’s surface warmer than it would be without an atmosphere.
There I said it….
BUT, and you knew there would be a BUT, and it is a BIG but. That statement is a false comparison, leading to a false conclusion, that there is therefore a greenhouse effect, when there is not.
Earth without an atmosphere (let us substitute the moon for our purposes here) would be seen as an object in space at an average temperature of -18C. Yes / No?
I will assume the answer is yes.
Earth with an atmosphere is seen as an object in space at an average temperature of -18C. Yes / No?
Again, I will assume the answer is yes.
These are two different systems, reaching the same answer. How? Well, in the first case, of no atmosphere (and therefore no oceans), the surface emits IR directly to space. Obviously a no atmosphere earth is not a black body, BUT, somehow it does get the same answer. Well, the moon does. The point though is that THE SURFACE emits IR directly to space.
In the second case, because of the presence of an atmosphere earth’s surface can not emit unhindered to space. Therefore a temperature gradient must develop through the depth of the atmosphere, so that it is seen as an object in space at an average of -18C. This means that in the case with an atmosphere the effective surface of emission IS NOT the surface, but that that “surface” must be at altitude within the atmosphere. Earth has mass, so it can have a temperature. You have to have mass to have a temperature. Space has no mass so it has no temperature, or rather space is temperature neutral. Thermal radiation passes through space, it does not warm space, that is a false notion. Obviously otherwise earth would not be warmed by sunlight, according to the inverse square law. Things in space can have a temperature, but space does not. In effect, earth’s surface is hot and space is not. Therefore a temperature gradient has to form in earth’s atmosphere, according to the laws of thermodynamics. Hot to cold is the same as hot to nothing in this case. So, in this case the earth’s surface is the bottom of the system, whereas in the first case with no atmosphere the surface is the complete system. This means that in the case with an atmosphere, according to the laws of thermodynamics, and the fact the oceans have a massive heat capacity, earth’s surface HAS TO BE WARMER than if there were no atmosphere. This is NOT proof there is a greenhouse effect as so many seem to currently think it is. It is merely that “we” are falsely comparing two different parts of two very different systems and reaching the wrong conclusion, because heat ALWAYS goes from hot to cold, or in this case heat goes from hot to not.
Most do not seem aware “we” are comparing the whole system, of a surface with no atmosphere, to the bottom (only) of a different system when we say,
“The presence of an atmosphere makes earth’s surface warmer than it would be without an atmosphere.”
BUT, we are, and “we” are drawing the wrong conclusions if we think such a false comparison is proof there must be a greenhouse effect.
Mike, the three models you try to describe in this post,
http://scottishsceptic.co.uk/2014/11/27/simplified-atmospheric-model/
may well have been better as follows……
Three very different systems in which the surfaces are not directly comparable, because they are different systems, and therefore the surfaces are different parts of the different systems.
1) B/B predicted surface of no mass temperature.
High, 123C, low -273C.
Average surface temperature. -18C.
Hypothetically seen as an object in space. -18C
2) The moon has a subsurface with mass and therefore heat capacity, and a very thin, albeit negligible atmosphere.
http://www.space.com/18175-moon-temperature.html
Moon surface temperature, high 123C, low -152C (-247C in a crater).
Average surface temperature. -15C.
Moon as an object in space. -18C.
3) Earth has oceans, a far, far thicker atmosphere, AND, the transport of latent heat losses and gains within the atmosphere.
http://www.space.com/17816-earth-temperature.html
Earth surface temperature, high 70.7C, low -89.2C.
Average surface temperature. between 25 to 28C.
Earth as an object in space. -18C.
That would be the next step – to go from a theoretical planet with a uniform temperature to a real planet.
Off the top of my head, the first would be a stationary totally insulating planet, the next would be a totally insulating planet surface rotating (where heat capacity starts to be important) and then I suppose trying to reconcile the real data we have for our planet with the various models.
However, if you reject the idea of black body radiation, you won’t like any of these. Moreover, all I will get is another surface temperature to plug into more complex heat flows.
A great discussion, but I have a different question which perhaps someone might answer for me. If a CO2 molecule goes into the atmosphere would it effect Photosynthesis or Global Warming more. Which would it effect first and more powerfully? Just assuming it could effect warming for a moment.
I can just see this CO2 molecule, and oh yes, only the one made by man (1 in 88,590 ) going into the atmosphere and saying to heck with creating plants/food I am going to warm the planet.
Well, if a plant uses the CO2 molecule then there will be more life on the planet, and that is happening.
http://www.co2science.org/data/plant_growth/plantgrowth.php
ie,
http://onlinelibrary.wiley.com/doi/10.1002/grl.50563/abstract;jsessionid=DACBD1BD9E71E340FE6A63DE7F7F47F1.d03t04
However, supposed man made, due to our CO2 emissions, global warming is NOT occurring..
http://www.collective-evolution.com/2013/04/15/scientists-baffled-as-report-proves-global-warming-has-stopped/
Does that answer the question Gary?
Nickreality, evaporation is another red herring. If warmer air does not lead to a warmer surface of the Earth then please explain why people bother to measure the temperature of the air with (mostly dry) thermometers.
Warmer air makes the surfaces of thermometers warmer.
How can faster air molecules (warmer air) fail to make anything they hit warmer than they would be if hit with slower air molecules?
Mikky: The weather service also measures dew point or wet bulb to determine the humidity, i.e. water content of the air. There’s no red herring about it. When air absorbs water it gains a large amount of heat without increasing its temperature. It’s how evaporative coolers work. It’s how sweat cools your body. It’s how the earth’s water vapor/clouds/precipitation cycle, which IPCC AR5 TS.6 admits to low certainty, modulates/moderates and controls the climate.
Derek Alker says:
26th November 2014 at 10:38 am
Derek’s post is essentially correct.
The additional warmth above the S-B expectation is a result of the mass of the air and oceans but bear in mind that the mass of the air also fixes the energy content of the oceans so in the end it is all about atmospheric mass.
When I learned about the Greenhouse Effect in the 1950s my understanding was that it was known to be all about mass at that time but the knowledge seems to have been lost.
There is a Greenhouse Effect but it is caused by mass and not radiative characteristics.
Variations in convection cancel out the thermal effect of compositional variations including radiative capability.
Stephen Wilde writes –
“but bear in mind that the mass of the air also fixes the energy content of the oceans so in the end it is all about atmospheric mass.”
!!! If I run a hot bath my bathroom is hot, if I run a cold bath my bathroom is cold…
Your bathroom is an enclosed space which does not allow free convection.
Much like earth’s atmosphere then…
btw – It seems rather ridiculous to suggest that earth’s atmosphere’s heat capacity determines the oceans heat capacity as you seem to suggest when you write,
” the mass of the air also fixes the energy content of the oceans so in the end it is all about atmospheric mass.”
when the oceans heat capacity is many, many, many times larger, about a thousand times (from memory)….
Heat capacity, the simple version – Slow to warm, slow to cool. Therefore, the oceans determine the atmospheres temperature, far more than the other way around.
Hence GMT is effected by the warm and cool cycles of the PDO, even on quite short time scales, such as 1998 for example…
You think an atmosphere open to space is similar to an enclosed bathroom in terms of convection ?
I think not.
As regards the heat capacity of the oceans you are right but that isn’t the point. The oceans will only absorb solar energy to the extent that the weight of the atmosphere permits them to do so.
If there were no overlying atmosphere then the oceans would immediately turn to vapour, freeze and fall to the ground as a solid.
The heavier the atmosphere the more energy the oceans need to hold before evaporation can occur because the amount of energy required by the latent heat of vapourisation is related to the weight of the atmosphere
However you are also right to say that once the weight of the atmosphere has determined the energy content of the oceans then the oceans determine the temperature of the air.
Pingback: Cloud feedbacks | Scottish Sceptic
You are wrong about greenhouses. They work by the glass reflecting the IR (long wave) radiation back inside, where it can be absorbed by the air inside, or by any other body. This effect will work even without air.
I read a paper some time ago showing it was reduction in convective flow. I see Roy Spencer has something suggesting it is IR. The simple way to test the two is to have a greenhouse with a /| type window which is open enough to let out air but not enough to stop blocking the window.
If opening the window doesn’t affect the temperature, then you are right – convection doesn’t play any role in keeping a greenhouse warm – leaving just IR and conduction.
Next time I’m at my mother’s I will have to try it – although she won’t be very pleased because she’s got one of these automatic window opener things which I will have to detach to close the window on a sunny day.
Daryl, why then are the surroundings of a greenhouse cooler than the greenhouse when the greenhouse is emitting MORE IR than it’s cooler surroundings, but both are being wamred by the same amount of solar input? Surely the answer is the most powerful cooling mechanism has been reduced / stopped within the greenhouse and thus it is warmer than it’s surroundings.
Your “explanation” does not make sense.
The greenhouse is radiating more IR because it is hotter than its surroundings. It is hotter because of the accumulated heat it has obtained.
Yes Daryl,
“The greenhouse is radiating more IR because it is hotter than its surroundings.”
For the same solar input……
WHY then are the surroundings cooler?
It can not be because of “trapped” radiation, because as you state correctly the greenhouse is emitting more radiation BECAUSE it is hotter. Daryl, sorry to state the blitheringly obvious, but “trapped” IS NOT emitting MORE…..
The answer has to be that the surroundings are cooled by something more powerful, ie, conduction and convection of sensible and latent heat of water vapourisation losses to the rest of the atmosphere, that has been stopped in the enclosed greenhouse.
This can be proved by opening the doors and windows of the greenhouse and watching the temperature in the greenhouse fall to the temperature of the surroundings…
If you leave a window open, you will loose heat because of convection. The hot air will escape. The proper way to perform the experiment is to evacuate the air entirely from the greenhouse. That is highly impractical, so the closest thing is to perform a smaller scale experiment in the laboratory with an evacuated vessel.
You also fail to account for the other gasses along with CO2. A CO2 molecule upon receiving a photon, may re-radiate the energy, or, if it happens to contact another molecule (especially H20), will transfer the energy to the surrounding atmosphere.
I’ve used CO2 as an example but it applies equally to H20 or any other IR interactive gas.
And the reason I added the footnote about “If necessary consider it in an inert atmosphere of transparent gas” … is because temperature is pretty meaningless for a single atom – and the only way it maintains its temperature is by transfer too and from the surrounding gas.
There are few interactive gasses with the efficacy of CO2. Global warming does not apply equally to CO2 and H2O, because they have different modes of absorption. Temperature IS meaningful for a single atom, because you can measure the kinetic, ionic and chemical energy of that atom compared to a similar atom nearby.
After reading and participating in a few blogs I stepped back for a moment and realized there was something amiss. It appears to me there are two theories regarding the mechanism of CO2/GHG/atmospheric heating: theory A based on UV on the higher energy side of visible light and theory B based on IR on the lower energy side of visible light.
Theory A
High energy UV (UV-A, UV-B, damages eyes, burns skin) of appropriate frequency knocks electrons out of orbit in CO2 molecules. (Einstein’s photoelectric effect) When these electrons return to their stable orbits photons with energy diminished by the work function they are coincidentally atuned to heating water molecules ala microwave oven. This leads to a general heating of the atmosphere, which heats the ocean (unlikely when opposed to evap) which outgasses more CO2 leading to a positive feedback loop of disputed magnitude. The radiative feedback loop pf IPCC AR5. No S-B or GHE. BTW I posit this theory in my writerbeat posting and after 700 plus reads have yet to be chastised or corrected.
Theory B
IR from the sun (SWIR?) heats objects on the surface of the earth (oceans, too?) which radiate LWIR per S-B (does water follow S-B?) which is both trapped by the atmosphere (GHE) yet carries energy out of the atmosphere to maintain the balance. CO2 absorbs this LWIR reducing the heat leaving the atmosphere (blanket, resistors) and re-back radiates heat from a colder troposphere to a warmer surface and maybe amplifying the energy in the process.
One of these theories goes home with the 2015BMW X-5, the other with a gift box of sausage and cheese.
Do-do-do-doo-do-do-do (Jeopardy)
Theory A – yes UV, yes photoelectric effect – general heating yes, heats oceans – safer to say “warms” outgassing CO2 yes, feedback???
Theory B – IR yes, heats object on surface – a lot of heating to atmosphere, radiates yes, water S-B yes but strongly angle dependant. CO2 absorbs yes (but water more important).
First one is interesting as you mention heating oceans. Warmer oceans => more water evaporated => more clouds => more blocking of sun => colder. And the effect of blocking cloud is far far stronger than CO2, so we’ve massive feedbacks which act to tend to push system back down in temperature and catastrophic warming is completely out of the question.
Pingback: Simplified atmospheric model | Scottish Sceptic